More on the behavioral characteristics of various compressors
By Richard Hawkins, MACS contributor
Last week’s article ended with a slide from my class at the MACS Training Event & Trade Show. If you haven’t already read that article, you may want to do so. That slide had the following question in it: Finally, with a 30 to 32 PSI low side, why is the suction line temperature 65° F? Please see picture #1.

Picture 1: : The slide referenced above from last week’s article.
The answer to that question is: Superheat. Please see picture #2.

Picture #2: The answer with a bit more information.
Before explaining exactly what was going on with superheat causing the suction line to be 65° F, it is necessary make sure that everyone understands superheat. If you Google it, there are a few definitions that come up. The one that best applies to air conditioning is: Superheat is any temperature of a gas that is above the boiling point for that liquid. (For clarification purposes, the gas was a liquid before it boiled.)
The term superheat suggests something that is very hot, but that is not necessarily the case. A perfect example is this suction line we are discussing the temperature of. At 65° F, the refrigerant that was flowing through it contained a lot of superheat. However, 65° F is a temperature that is a few degrees below what is considered room temperature. It certainly is not hot.
T o better understand superheat, we will utilize an example of a moonshine still in operation. Please see picture #3.
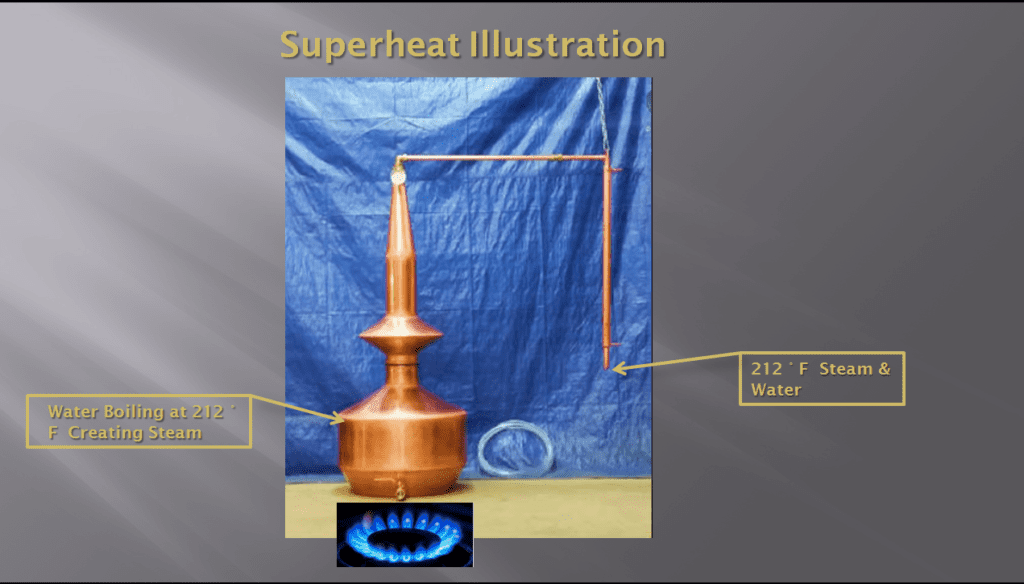
Picture #3: A moonshine still containing water with a hot fire under it.
Moonshine stills are used to distill ethyl alcohol (aka liquor) from fermented mash. Ethyl alcohol has a boiling point of 173.1° F. In the interest of keeping things legal, we will use water instead of mash. In this example the still has a water level in it that is indicated by the arrow extending from the box which says, “Water Boiling at 212° F Creating Steam”. It also has a hot fire under it. Water boils at 212° F at sea level, so we will pretend we are at Daytona Beach, FL.
When the water in the still reaches 212° F, some of it will start to turn to steam. The pressure generated by the boiling will cause the steam to rise upward to the higher portions of the still. The temperature of the higher portions of the still are below 212° F because there is no additional heat being applied. As a result, some of the steam will condense back to water and will fail back down to the lower portion of the still. Some of the steam will also continue its journey upward and through the two 90° bends in the piping.
As this steam continues its journey, more of it will condense back into water and both water and steam will exit from the pipe where it is labeled: “212 ° F Steam & Water”. This steam that exits is called saturated steam. It is called saturated, because at 212° F there is no additional heat being added as the steam makes its journey through the still.
Now it is time to illustrate how steam becomes superheated. Please see picture #4.

Picture #4: The still with additional heat being applied to it.
In picture #4, two propane torches are being used to apply additional heat to the upper portion of the still. This additional heat raises the temperature of the steam from 212° F to 214° F. As a result, we now have 214° superheated steam exiting the still. This steam contains 2° F of superheat.
If we apply additional heat, more superheat can be added to the steam. Please see picture #5.
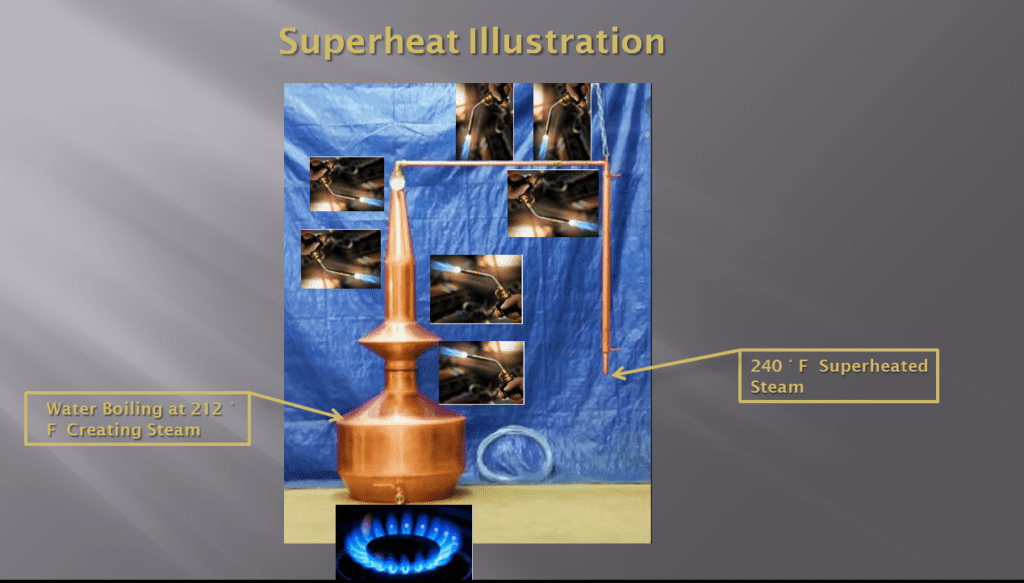
Picture #5: The still with even more heat being added.
In picture #5, five more propane torches have been added to apply even more heat to the still. The result is superheated steam exiting the still at 240°F. This steam contains 28° F of superheat.
Check back in next week and we will apply the concept of superheating to an air conditioning system and explain why that suction line temperature was at 65° F when the low side pressure was in the 30 to 32 PSI range.
Do you like good educational technical information like this? Join MACS as a member for access to much more.

Leave a Reply